Overview of Libtayo, First New FDA-Approved Drug for Metastatic Cutaneous Squamous Cell Carcinoma (CSCC) | Everyday Health
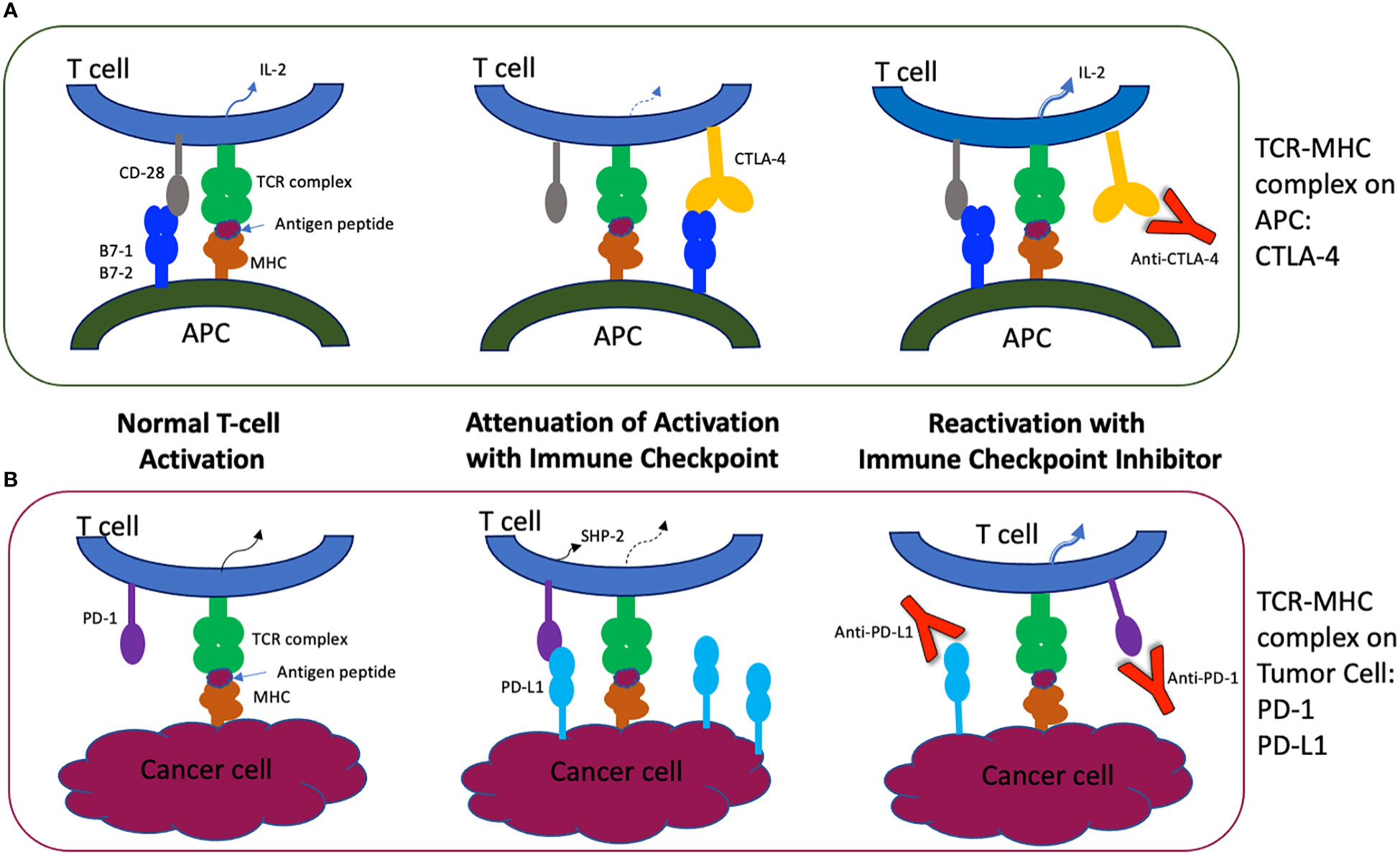
Frontiers | Neuro-ophthalmic complications of immune checkpoint inhibitor therapy: Current status and future directions