101831-37-2 | Diclazuril | 2,6-Dichloro-α-(4-chlorophenyl)-4-(4,5-dihydro-3,5-dioxo-1,2,4-triazin-2(3H)-yl)-benzeneacetonitrile; Clinacox; Nuoqiu; P 64433; R 64433; Vecoxan; | C₁₇H₉Cl₃N₄O₂ | TRC

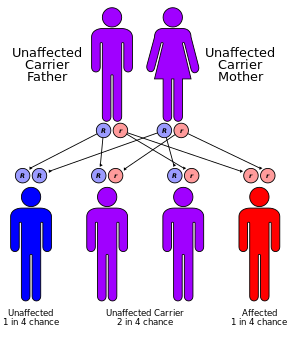
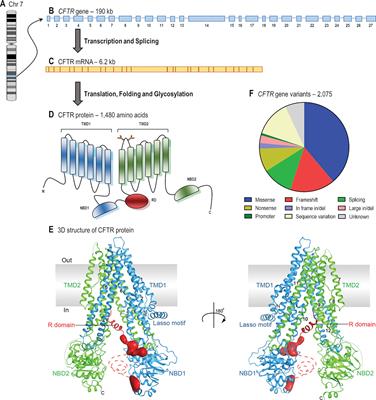
Lumacaftor and ivacaftor in the management of patients with cystic fibrosis: current evidence and future prospects - Kelly Kuk, Jennifer L. Taylor-Cousar, 2015

Design and Synthesis of Orally Bioavailable Piperazine Substituted 4(1H)-Quinolones with Potent Antimalarial Activity: Structure–Activity and Structure–Property Relationship Studies | Journal of Medicinal Chemistry
The impact of ivacaftor on sinonasal pathology in S1251N-mediated cystic fibrosis patients | PLOS ONE

Lumacaftor and ivacaftor in the management of patients with cystic fibrosis: current evidence and future prospects - Kelly Kuk, Jennifer L. Taylor-Cousar, 2015
The magnitude of ivacaftor effects on fluid secretion via R117H-CFTR channels: Human in vivo measurements | PLOS ONE
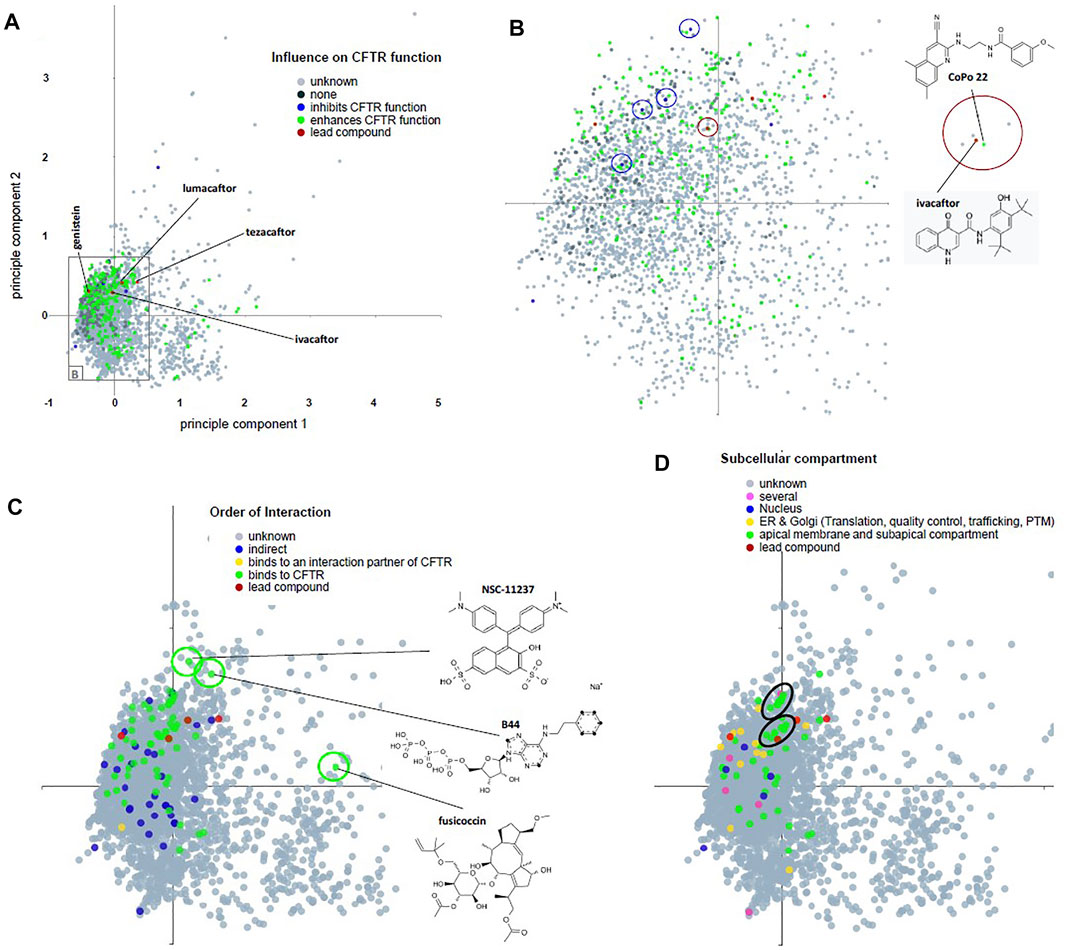
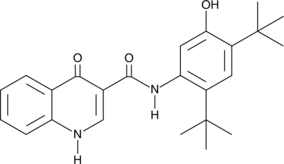
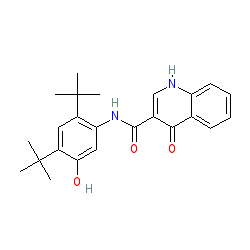
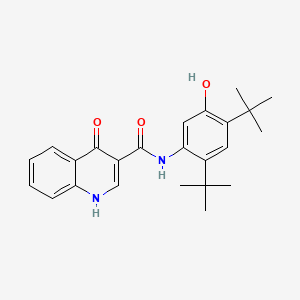
Frontiers | Comprehensive Analysis of Chemical Structures That Have Been Tested as CFTR Activating Substances in a Publicly Available Database CandActCFTR