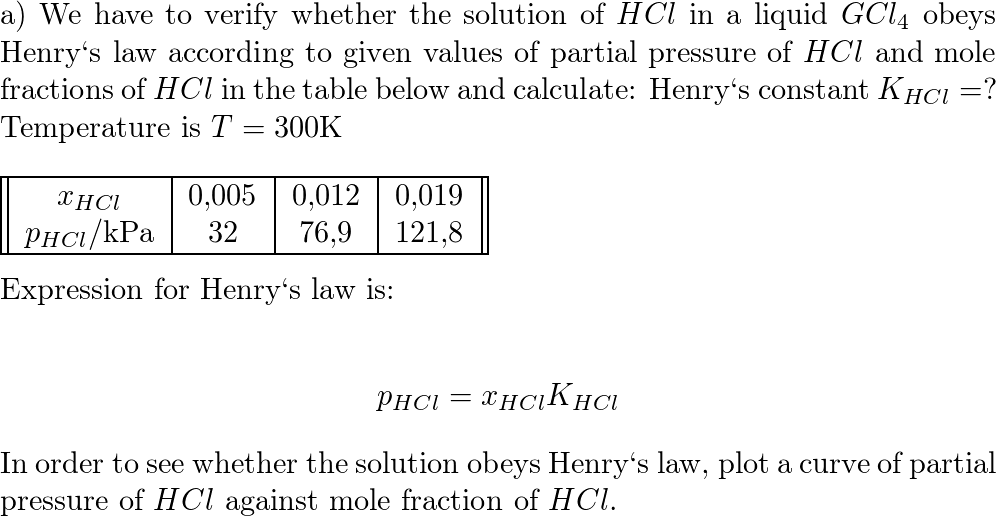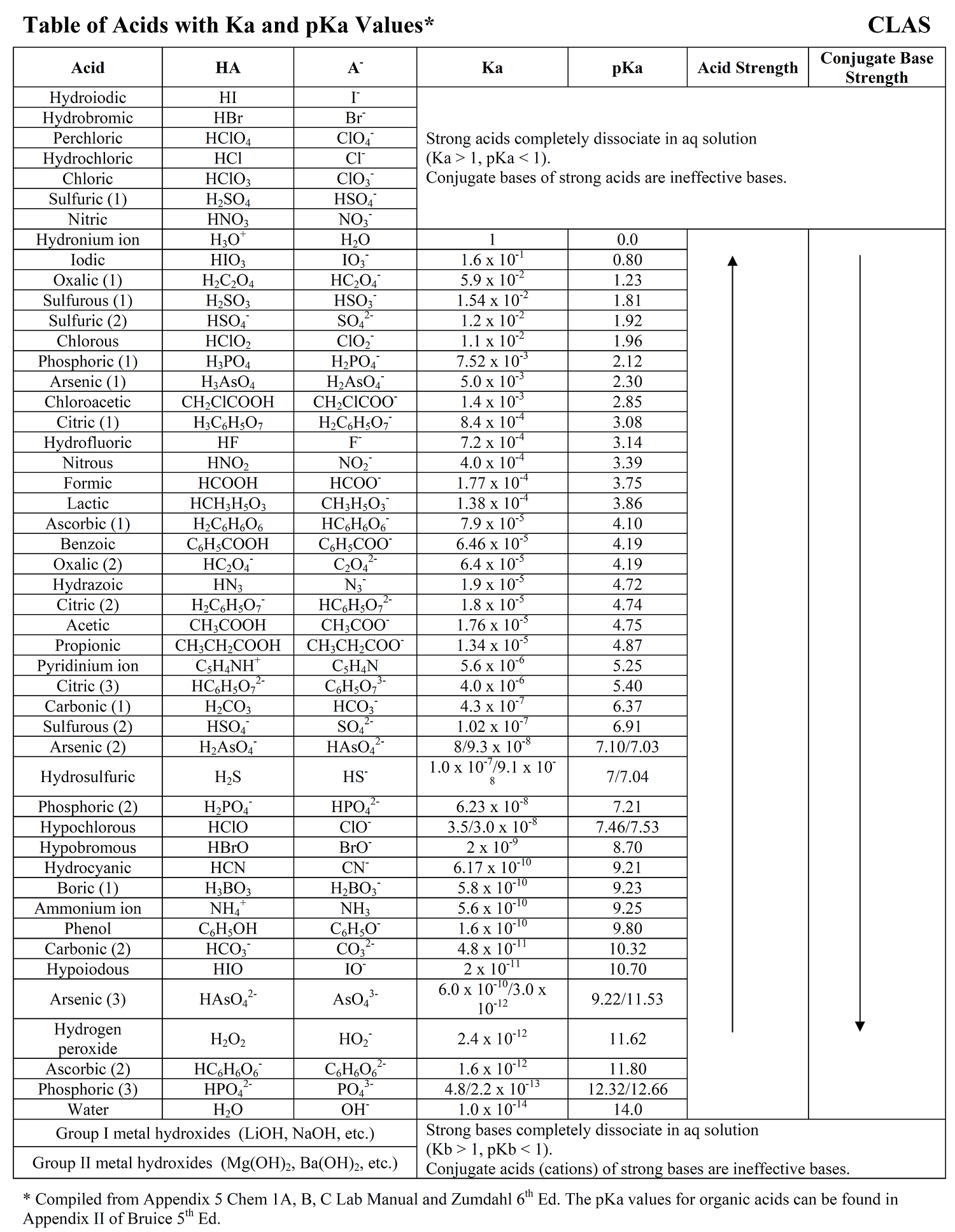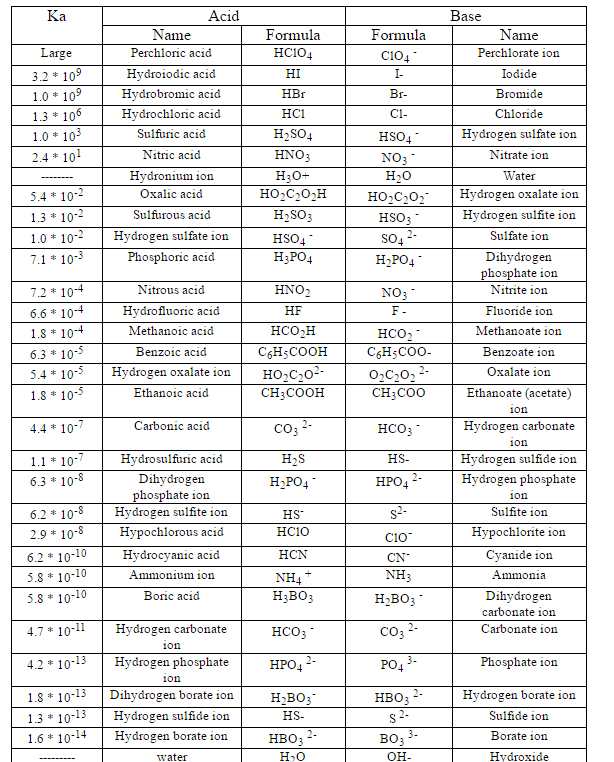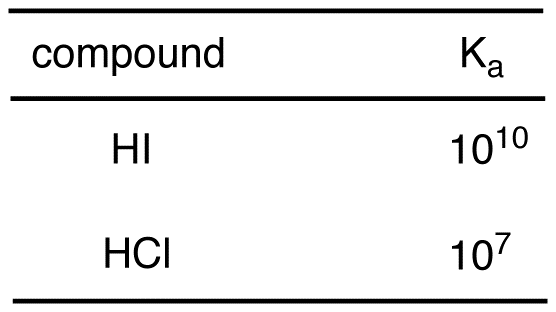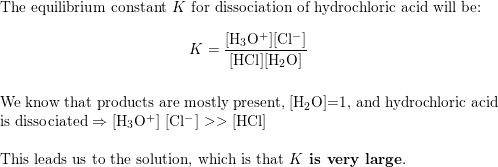
SOLVED: Determine the pH of a solution that is 0.00424 M HCl and 0.0228 M HClO2. The Ka of HClO2 is 1.1×10âˆ'2.

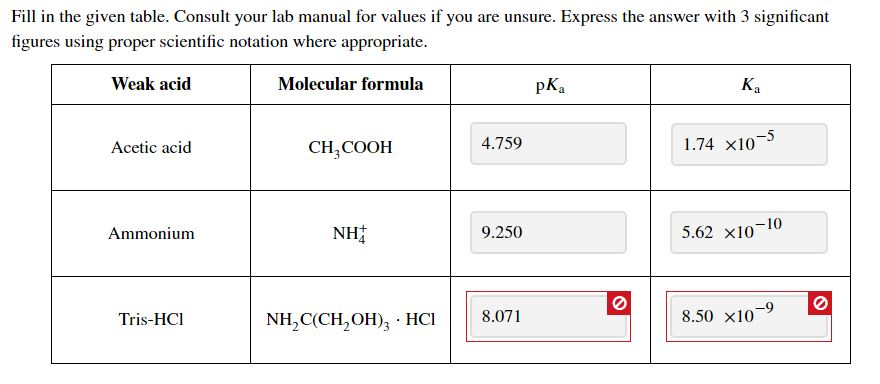
Ka for CH3COOH is 1.8×10^-5. find out the % dissociation of 0.2M CH3COOH in 0.1M HCl solution? - EduRev NEET Question

Binding Association Constants (Ka ) and Binding Sites (N) for Three... | Download Scientific Diagram

An aqueous solution of a metal bromide MBr2 (0.05M) is saturated with H2S . The minimum pH at which MS will precipitate is X ? Ksp for MS = 6.0 × 10^-21 . [
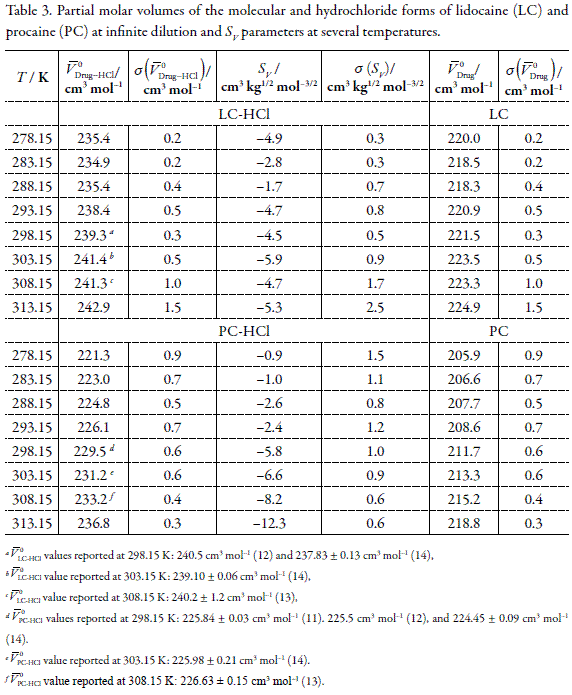
Apparent molar volumes of the anesthetic drugs procaine-HCl and lidocaine- HCl in water at temperatures from 278.15 to 313.15 K


![Solved HCl + H2O → H30+ + CH) = Ka = [H3O+] [C) = 1.3 x 106 | Chegg.com Solved HCl + H2O → H30+ + CH) = Ka = [H3O+] [C) = 1.3 x 106 | Chegg.com](https://media.cheggcdn.com/media/0fe/0fe53724-71a9-4b4c-a2f3-a791d50b9bb2/phpCUvOU0)