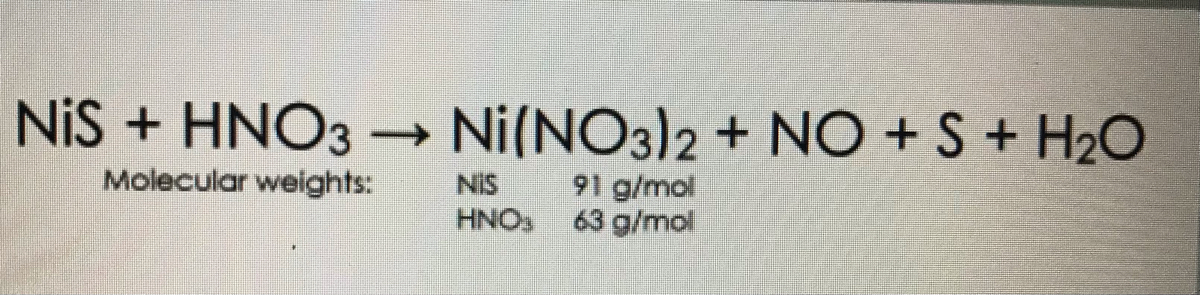
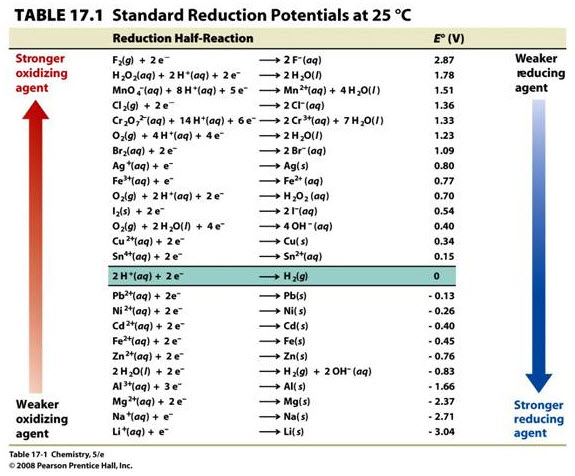
OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...

Salicylic Acid Nitration by Means of Nitric Acid/Acetic Acid System: Chemical and Kinetic Characterization | Organic Process Research & Development

Buy Nickel (Ni) ICP Standard Solution 1 gm/L in Diluted HNO3 traceable to NIST - NA – in India | Otto Chemie Pvt Ltd
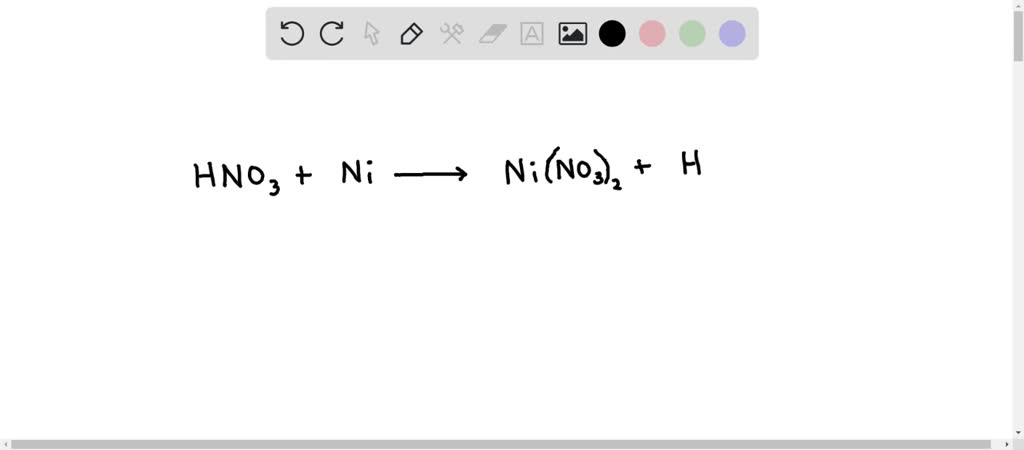
SOLVED: Write the balanced equation for the reaction of nitric acid and nickel metal reacting to form nickel(II) nitrate and hydrogen gas. Don't worry about states or making the subscripts subscript. Write
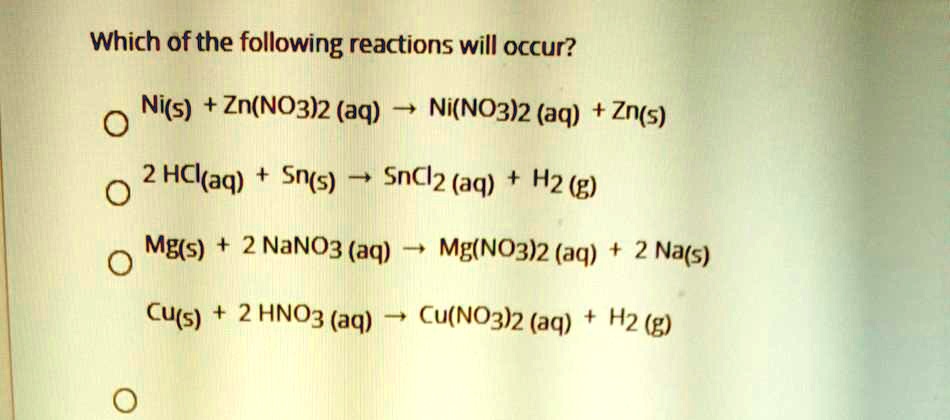
SOLVED: Which of the following reactions will occur? Ni(s) + Zn(NO3)2 (aq) â†' Ni(NO3)2 (aq) + Zn(s) 2 HCl(aq) + Sn(s) â†' SnCl2 (aq) + H2 (g) Mg(s) + 2 NaNO3 (aq)

How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3? - India Site

science chemistry solubility reaction nickel oxide nitric acid | Fundamental Photographs - The Art of Science

Process Intensification in Nitric Acid Plants by Catalytic Oxidation of Nitric Oxide | Industrial & Engineering Chemistry Research
![PDF] Studies on Ni-Sn intermetallic compound and P-rich Ni layer at the electroless nickel UBM-solder interface and their effects on flip chip solder joint reliability | Semantic Scholar PDF] Studies on Ni-Sn intermetallic compound and P-rich Ni layer at the electroless nickel UBM-solder interface and their effects on flip chip solder joint reliability | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/dd3b9c617b9af5ce8d4299a0c71bb33d2e456dc1/2-Table1-1.png)