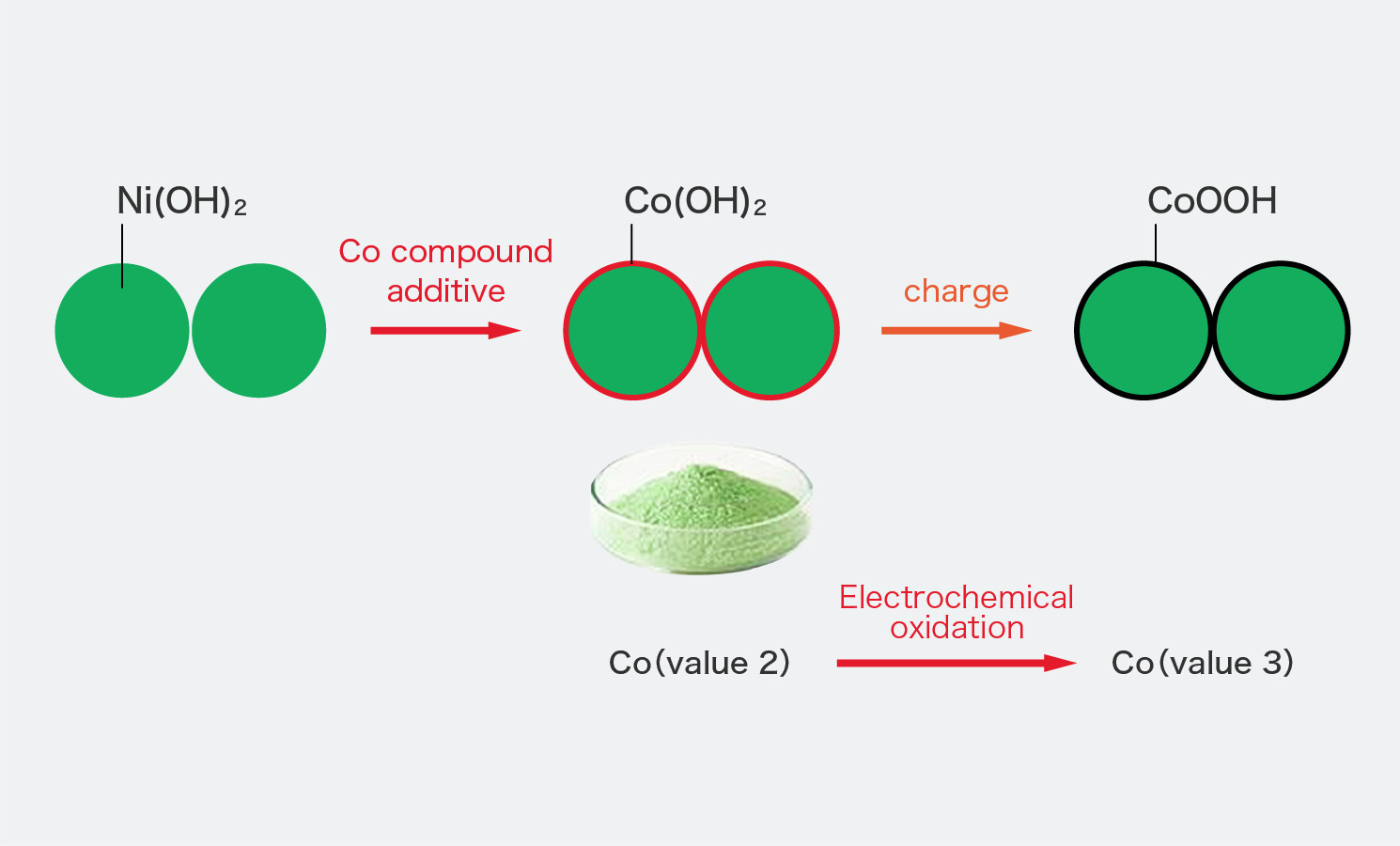
Figure 3 from Electrochemical Performance of β-Nis@Ni(OH)2 Nanocomposite for Water Splitting Applications | Semantic Scholar

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
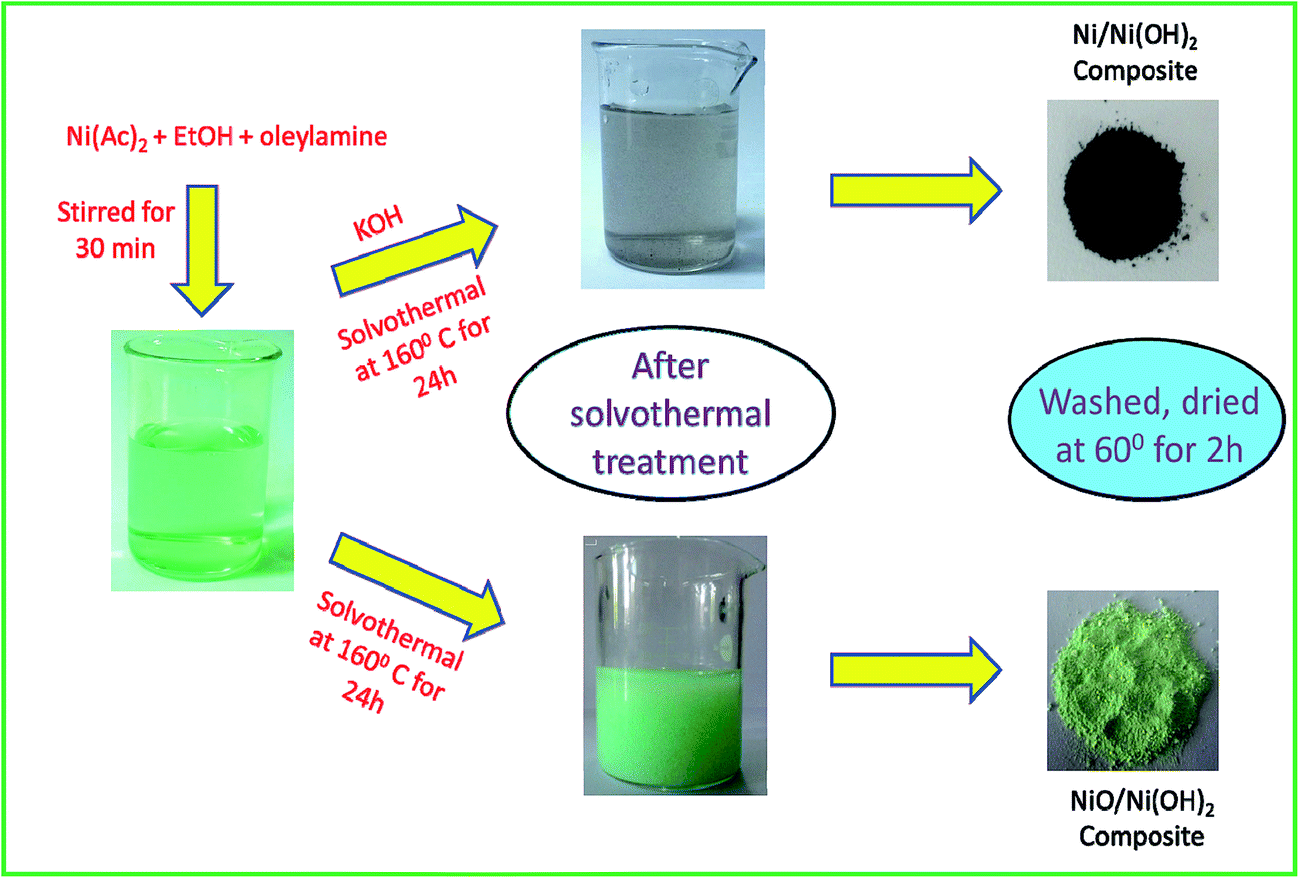
One step synthesis of Ni/Ni(OH) 2 nano sheets (NSs) and their application in asymmetric supercapacitors - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26584G

Chemistry lovers - #Metal hydroxides are hydroxides of metals. Metal hydroxides are also known as strong bases. Many common metal hydroxides are made up from hydroxide ions and the ion of the

Direct preparation of Al-substituted α-Ni(OH)2 from Al-containing salt solution by immersing method - ScienceDirect

5 Six methods of preparing Ni(OH) 2. (a) Basification of a nickel(II)... | Download Scientific Diagram
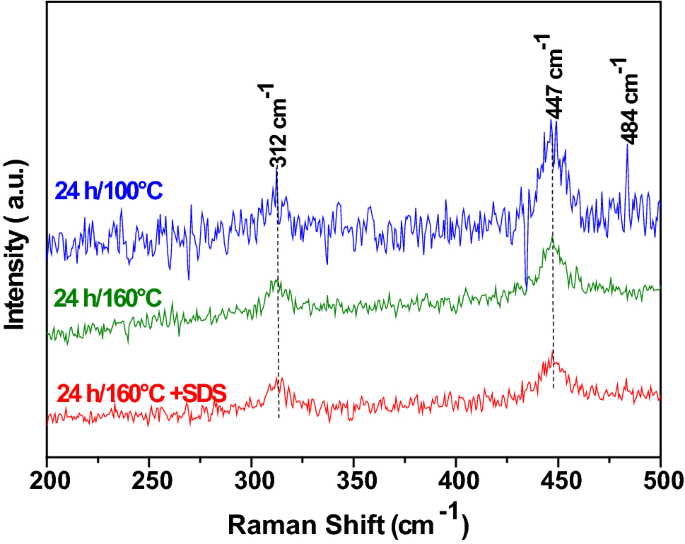
Electrochemical measurements of synthesized nanostructured β-Ni(OH)2 using hydrothermal process and activated carbon based nanoelectroactive materials | SN Applied Sciences
![1. Given the following equilibrium constants, calculate the solubility (moles/L) of Ni(OH)2(s) in a solution that has a fixed [OH-] of 3.2x10-7M Ni (OH)2(s) Ksp. - ppt download 1. Given the following equilibrium constants, calculate the solubility (moles/L) of Ni(OH)2(s) in a solution that has a fixed [OH-] of 3.2x10-7M Ni (OH)2(s) Ksp. - ppt download](https://slideplayer.com/15074812/91/images/slide_1.jpg)
1. Given the following equilibrium constants, calculate the solubility (moles/L) of Ni(OH)2(s) in a solution that has a fixed [OH-] of 3.2x10-7M Ni (OH)2(s) Ksp. - ppt download
![The Role of the Redox Non‐Innocent Hydroxyl Ligand in the Activation of O2 Performed by [Ni(H)(OH)]+ - Kim - 2023 - Chemistry – A European Journal - Wiley Online Library The Role of the Redox Non‐Innocent Hydroxyl Ligand in the Activation of O2 Performed by [Ni(H)(OH)]+ - Kim - 2023 - Chemistry – A European Journal - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/5a5d2b1b-25f4-4e6d-a83c-c65beeb759da/chem202203128-fig-0003-m.jpg)
The Role of the Redox Non‐Innocent Hydroxyl Ligand in the Activation of O2 Performed by [Ni(H)(OH)]+ - Kim - 2023 - Chemistry – A European Journal - Wiley Online Library

XRD patterns of (a) In(OH)3, (b) Ni(OH)2, (c) Ni(OH)2/In(OH)3, and (d)... | Download Scientific Diagram
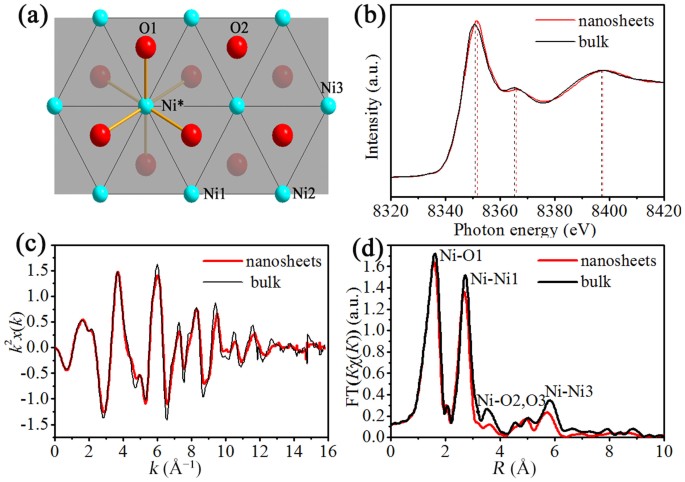
Ultrathin Nickel Hydroxide and Oxide Nanosheets: Synthesis, Characterizations and Excellent Supercapacitor Performances | Scientific Reports
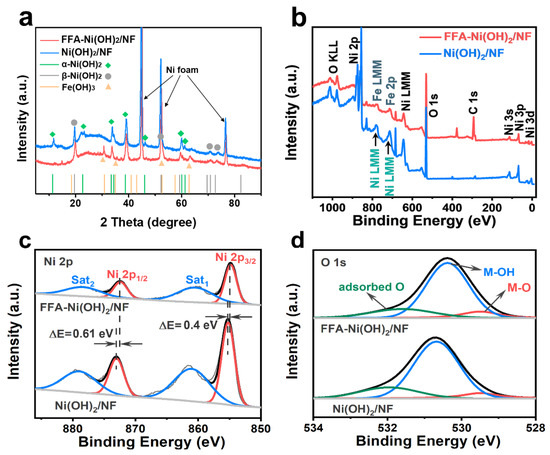
Crystals | Free Full-Text | Ferrocene Formic Acid Surface Modified Ni(OH)2 for Highly Efficient Alkaline Oxygen Evolution
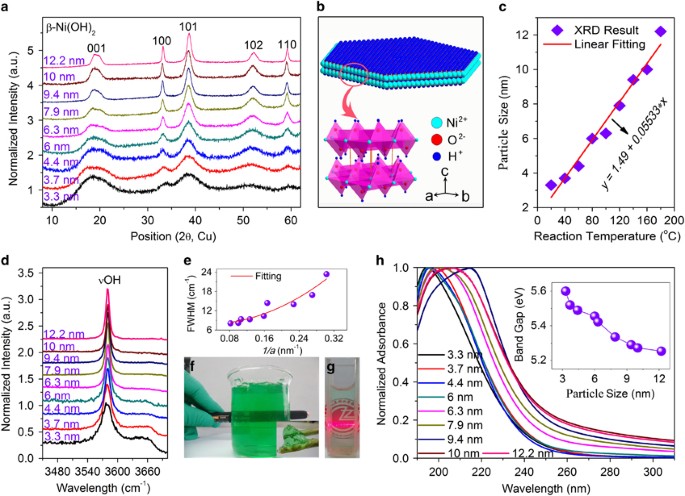
Ultra-small, size-controlled Ni(OH)2 nanoparticles: elucidating the relationship between particle size and electrochemical performance for advanced energy storage devices | NPG Asia Materials

K of a salt Ni(OH), is 2 x10-15 then molar solubility of Ni(OH), in 0.01M NaOH is :- (1) 2 x 10-15 M (2) 21/3 x 10-5 M (3) 2 x 10-11 M (4) 107 M